The mechanism of hydrogen inhalation
Generating hydrogen
Hydrogen exists as a lightest, odorless, colorless, tasteless gas and in a water form in the earth.
75% of the universe is composed of hydrogen.
The industry in hydrogen has developed from how to effectively gain the hydrogen gas, how to operate the machines including vehicles, trains, house and other small appliances using it, how to add value on the hydrogen gas for food or medicine, how to safely store hydrogen gas, and how to deliver these all factors work around in the economic system.
There are broadly three method of separating hydrogen and oxygen gas: hydrogen as a byproduct, natural gas reformation, and water electrolysis technology.
Hydrogen Byproduct Method and natural gas reformation is relatively cheap. These methods are being used in the industrial field, so it is not appropriate for inhalation in quality and environmental aspects.
Water electrolysis technology includes alkali electrolyte method and polyelectrolyte (a.k.a. Proton Exchange Membrane (PEM)) method. With this said, polyelectrolyte method is the safest way to produce hydrogen gas frequently inhaled at hospitals and homes. With this method, the water is separated into Hydrogen and Oxygen gas via PEM that uses pure white gold, titanium, so the downside is that it is expensive. Still, it allows us to inhale hydrogen with a purity of 99.9995% and was acknowledged as a technology with vast potential. On the other hand, the Alkali Electrolyte Method is a relatively old method. With this method, NaOH, an electrolysis catalyst, or calcium hydroxide, is added to the water. Even if there’s a filter to filter out the odor and contaminants, this method is not quite suitable to be used for inhalation purpose.
Is hydrogen inhalation safe? Yes.
Some say the explosive risk of the hydrogen inhalation device. When we investigate on the explosion,
explosion may be categorized to either physical and chemical explosion.
For the physical explosion to occur, hydrogen must be stored in a storing tank and has to be expanded with high pressure and there must be a crack on the storing tank. Hue Light’s hydrogen inhalation device emits the gas as soon as it is generated, so it does not store the hydrogen inside the device. So, there’s no storing tank inside the device.
Theoretically, chemical explosion occurs as a combustion reactivity when all of the following criteria are met: leak > gas cloud > ignition source. It is very difficult for the hydrogen to form a gas cloud because it easily diluted to the air, so it would be difficult for all aforementioned criteria to be met. However, the user should always be cautious not to ignite a cigarette, lighter and other fire igniting source in front of the opening where the hydrogen gas comes out.

Hue Light’s Technology
The advanced features of our hydrogen inhalation device are as follows.
-
1 We have the cells that generate from 1,350 ml~3,000ml of gas per minute.
The medical grade devices and devices used in the clinical researches have used high capacity hydrogen-oxygen generating devices.
This technology is advanced than the cells that only generate less than 1,000 ml of gas per minute. -
2We have developed PEM cell using the pure catalysts including Platinum and Iridium;
the efficiency has doubled and it is optimized for the inhalation purpose -
3FDA, CE, RoHS complaint
-

Hue Light PEM Cell (1.5L~3.0L)
Disclaimer: Medical articles and academic information provided on this site are for educational purposes only and are not intended to diagnose, treat, or prevent any disease or to replace the advice of a physician. In addition, when the effects for a specific period, comparisons before and after product use, reviews of use, and end-of-life test results are specified, there may be different results for each user.
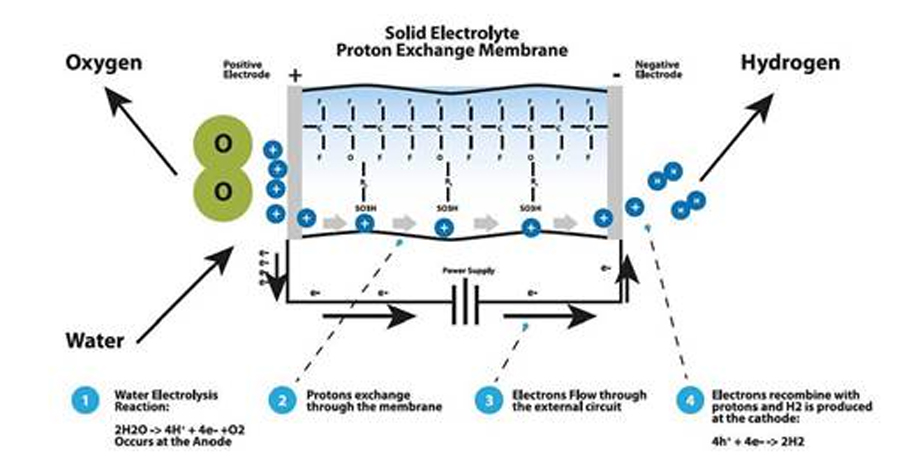
Polyelectrolyte system (proton exchange membrane, PEM) mechanism
Polyelectrolyte System uses the proton exchange membrane (PEM) to separate the distilled water. This allows the water and cation exchange and as the membrane acts as the cell’s electrolyte, so there’s no need for chemical electrolyte such as sodium hydroxide and/or potassium.
When current voltage gets permitted, the water is provided to positive electrode and/or oxygen electrode, so it becomes oxygen, proton and free electrons. Proton (H+ ion) passes through the PEM and moves to the hydrogen electrode and meets with the electrons to convert to hydrogen gas.
The reaction process is shown as follows.
1 2H2O → 4H+ + 4E- + O2
2 4H+ + 4E- → 2H2
The efficiency of Hue Light’s PEM technology is 2x better than
the existing technology and lasts longer.
Disclaimer: Medical articles and academic information provided on this site are for educational purposes only and are not intended to diagnose, treat, or prevent any disease or to replace the advice of a physician. In addition, when the effects for a specific period, comparisons before and after product use, reviews of use, and end-of-life test results are specified, there may be different results for each user.
-
Product
-
Medical engineering
research -
Information
search -
References
-
Hue Light Site
-
Immune Care
Center -
Hue Light USA